For over a century, there were only two drugs against Alzheimer’s. And neither was too effective. So, it’s unsurprising that companies, doctors, and patients were eager to find a new magic drug. That drug arrived in 2021. But was it as effective as they said?
A few years ago, there were 2 options to treat Alzheimer’s disease. For mild Alzheimer, there were cholinesterase inhibitors, like Donepezil. For people with advanced Alzheimer, there was memantine. Both had limited effect and only worked for a few months.
In 2021, after 18 years without any breakthroughs, Biogen announced a new promising drug: aducanumab. Its commercial name: Aduhelm.
In June 2021, the FDA green-lighted its approval
And it was the first treatment approval for Alzheimer’s in 18 years. It was an accelerated approval, though. What does that mean? It’s a way to approve some drugs before proving they actually work. They can do that on account that:
- that drug treats a serious condition
- there are no other effective therapies
- the drug will likely be effective because its mechanism should improve the disease
As a condition for the approval, the F.D.A. required Biogen to conduct another clinical trial in the next nine years. The goal of this trial would be to make sure the drug works once enough patients take it.
Why did they think aducanumab would work?
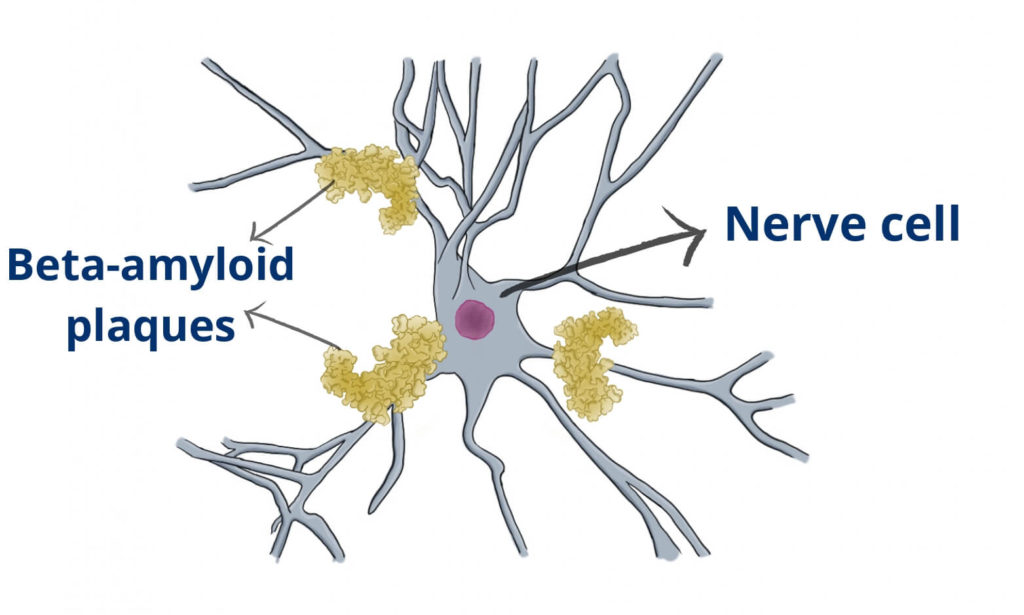
Because aducanumab was effective in clearing amyloid plaques from the patients’ brains.
Many years ago, doctors found microscopic plaques in Alzheimer’s patient’s brains. Healthy people, on the other side, had none. So everybody assumed that these plaques were causing the disease.
After watching the preliminary trials with aducanumab, many experts started doubting. Were patients getting better?
For some reason, the drug was very good at clearing the amyloid plaques. Yet, patients weren’t improving after their plaques were gone.
It seemed like their memory and thinking stayed the same. Why?
Well, we now think that amyloid is a biomarker of the disease, but it is not necessarily its cause.
Imagine you have this problem: you spend too much money every month. So your house is full of bills from all the things you bought. Would you solve your problem by burning those bills? No, because they aren’t causing the problem. They are more of a by-product. Something similar happens with Alzheimer’s.
What did the experts think about aducanumab?
As you can expect by now, there was a lot of controversy around the drug’s approval.
After the FDA’s decision, three members of the FDA’s committee resigned. They believed they had been mocked.
The whole committee consisted of 11 members. None of them considered the drug ready for approval. 10 members voted against it and the other one was uncertain. And despite this fact, the FDA decided to approve it. Then, what did they need the experts for?
The committee didn’t find the evidence convincing. It was not clear that the drug could slow down the disease. On top of that, Aduhelm could cause serious side effects, like brain swelling and brain bleeding.
Here are some things the experts said:
· “Biomarker justification for approval in the absence of … clinical benefit after 18 months … is indefensible.” Dr. David Knopman, neurologist at the Mayo Clinic.
· “This might be the worst approval decision that the F.D.A. has made that I can remember” Dr. Aaron Kesselheim.
· “Approval of a drug that is not effective has serious potential to impair future research into new treatments that may be effective,” Dr. Joel Perlmutter, neurologist at Washington School of Medicine. He was the first to resign from the committee.
· “The implementation of aducanumab therapy will … cost billions of dollars, which may be better spent in either developing … other therapeutic interventions” Dr. Perlmutter.
The consequences
Even though all expert sciencists voted against this drug, the FDA chose not to listen. They thought they would know better.
The FDA’s goal should be evaluating risks and benefits so we don’t have to. However, their decision seems based on money, rather than facts.
Their action gives Biogen 9 years of cashing in. That’s a lot of time to make money.
And what happens after those 9 years? Nobody knows, but there’s a good chance that they will keep manufacturing it, even if they don’t prove it works.
The money that will go into these drugs (billions of dollars) could be deployed somewhere else. For example on better care centers, stress reduction programs, or to develop new drugs.
Their decision will probably set dementia research back by at least a decade.
Not only that, but it will also give patients a false sense of hope. Many patients will choose this expensive therapy, not knowing any better, all for the profit of a few. Instead, they could go on to promising trials.
Some final thoughts
Patients and their families search for any hope to combat this terrible disease.
Pharmaceutical companies have taken advantage of this situation offering relief and improvement.
But here’s the truth: many of these drugs are nothing more than a way to make money, failing to deliver results.
Despite its supposed potential, aducanumab has no real benefit in patients with Alzheimer’s.
The FDA should be ashamed of their decision. They ignored expert opinion and spent billions of dollars on an unproved drug.
You can read more about Alzheimer’s disease here.
Sources
· 3 experts resigning: https://www.nytimes.com/2021/06/10/health/aduhelm-fda-resign-alzheimers.html
· Aducanumab side effects: https://www.dukehealth.org/blog/what-you-need-know-about-aducanumab
· Effects of aducanumab: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2792897
Leave a Reply